/ BIOPHARMA COMPANY / IPO – JULY 2019 (NASDAQ: MIRM)
Mirum was founded upon a drug treatment that RiverVest helped develop through Lumena – a Fund II company acquired by Shire in 2014.
THE SNAPSHOT
- $120 million Series A in 2018
- IPO: July 2019 (NASDAQ: MIRM)
- Founded on a treatment co-developed by RiverVest
THE IMPACT
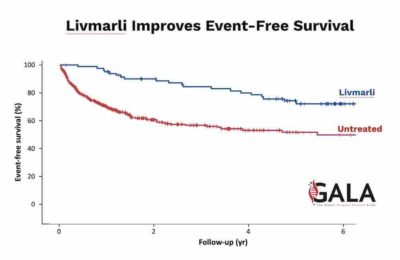
LIVMARLI, a minimally absorbed ileal bile acid transporter inhibitor, is the first and only FDA- approved medication for treating a rare liver disease that affects 2,000 to 2,500 children in the United States. Mirum is advancing LIVMARLI and volixibat, also acquired from Shire, across five additional late-stage trials for high-need rare cholestatic liver diseases.